Orbitals can be ranked in the increasing order of orbital energy as follows. The diatomic molecules having less than or equal to 14 electrons in all show s-p mixing.
8 2 Hybrid Atomic Orbitals Chemistry
From this situation we can infer that it is an sp hybrid orbital.

. Its a shell game to rationalise observationscommon sense. The same can be said for acetonitrile and allene. The energy of an electron in a single atom can be determined solely by the principal quantum number.
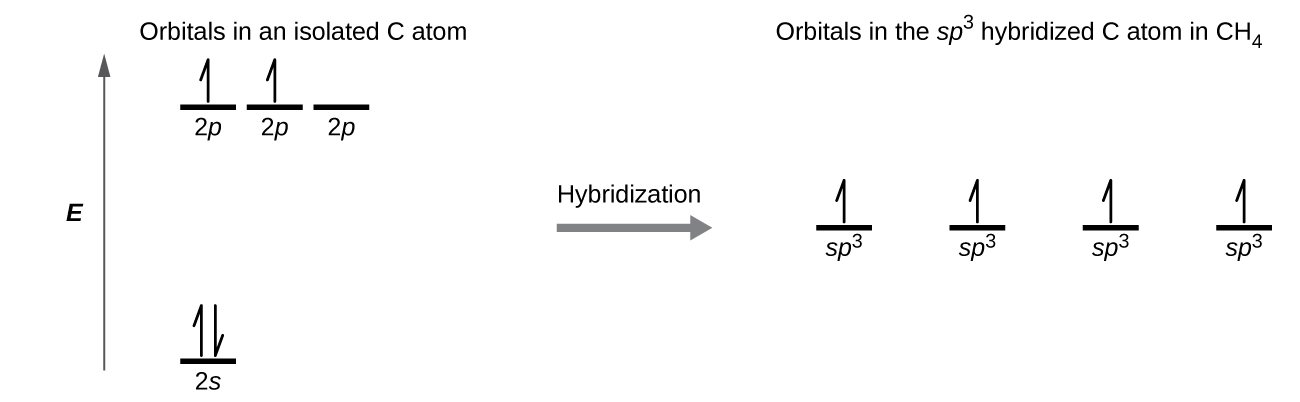
There is a total of four hybridized sp3 orbitals all of equal energy. In an sp3 hybridization colorredone s orbital is mixed with colorredthree p orbitals to form colorredfour sp3 hybridized orbitals. Energy changes occurring in hybridization.
When carbon atom bonding takes place between 1 s-orbital with two p orbitals then the formation of two single bonds and one double bond between three atoms takes place. The promotion of a Be 2s electron to a 2p orbital to allow sp hybrid orbital formation requires energy. The significance is that the energy gap between the hybrid orbital eg.
When Carbon is bound to two other atoms with the help of two double bonds or one single and one triple bond. Less than that of a p orbital but greater than that of an s orbital An sp orbital will have an energy in between those of the unhybridized s and porbitals. Examples of sp hybrid orbitals are acetylene acetonitrile and allene.
P orbitals have a higher energy than that of s orbitals. This will be discussed in the next section. This produces a stronger bond higher bond energy which offsets the energy required to promote the 2s electron.
CC-BY-NC-SA Kathryn Haas In the case of homonuclear diatomic molecules of the second row orbital mixing has important consequences for the energetic order of the σ g 2 p and π u 2 p orbitals. In compounds with sp hybrid orbitals many of them will have double or triple bonds. Answer 1 of 7.
It describes the angular momentum of electrons in the p orbital. The remaining p orbital remains unchanged and is perpendicular to the plane of the three sp 2 orbitals. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atoms nucleusThe term atomic orbital may also refer to the physical region or space where.
The energy of an SP orbital will be. There are two hands from the carbon atoms of acetylene. These sp3 hybridized orbitals are oriented with bond angle of.
Orbitals - Orbital Energy Orbital energy level. The letter p stands for principal. This type of hybridization involves the mixing of one s orbital and one p orbital of equal energy to give a new hybrid orbital known as an sp hybridized orbital.
In chemistry orbital hybridisation or hybridization is the concept of mixing atomic orbitals to form new hybrid orbitals with different energies shapes etc than the component atomic orbitals suitable for the pairing of electrons to form chemical bonds in valence bond theoryFor example in a carbon atom which forms four single bonds the valence-shell s orbital combines. P orbital is an atomic orbital having a dumbbell shape. Sp and sp 2 and an unhybridized p orbital is smaller than the spin paring energy and therefore electrons will be in the hybrid and unhybridized p orbital state before.
As the n in cespn grows larger we will have more p character and less s character in the hybrid orbital and as explained above it will consequently be higher in energy. Sp 3 hybridisation or Tetrahedral hybridisation. In localized valence-bond theory a process called hybridization is used which mathematically mixes atomic orbitals that are similar in energy but not equivalent are to.
By Hunds rule Key Notes In sp3 hybridization the s and the p orbitals of the second shell are mixed to form four hybridized sp3 orbitals of equal energy. These electrons occupy subatomic orbitals. The elongated sp hybrid orbitals have one large lobe which can overlap bond with another atom more effectively.
The process of mixing and recasting one s orbital and three p orbitals of the same atom having nearly the same energy to form four equivalent sp3 hybrid orbitals of equal energy having maximum symmetry and definite orientation in space are called sp3 hybridisation. In such molecules the energy difference between 2s and 2p orbitals is quite less and due to it the 2s orbital and 2p_z. This is referred to as the building up principle or the Aufbau Principle.
1s 2s 2p 3s 3p 3d the energy of an electron in multi-electron atoms depends on both on its principal quantum number n and. Sp 2 and sp 3 Hybrid Orbitals. In the ground state of an atom electrons prefer to fill accessible orbitals in increasing order of energy with the lowest energy orbitals being filled first.
In atomic theory and quantum mechanics an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. Answer 1 of 3. One p orbital can hold a maximum of 6 electrons.
Sp hybridization is also called diagonal hybridization. Mixing of orbitals with like symmetry affects the energies of molecular orbitals. Each hybridized orbital contains a single unpaired electron and so four bonds are possible.
Each sp hybridized orbital has an equal amount of s and p character 50 s and 50 p character. So an cesp hybrid orbital that has 50 s character will be lower in energy than an cesp3 orbital which only contains 25 s character. Hybridization of an s orbital with two p orbitals p x and p y results in three sp 2 hybrid orbitals that are oriented at 120 o angle to each other Figure 3.
Each of these hybridized orbitals have 25 s character and 75 p character calculated according to the proportion of sp mixing.

2 The Other Sp Orbitals Hold The Oxygen Lone Pairs
0 Comments